drops of bromine solution to test presence of alkene bones|alkyline bromination test : store In any event, the decolorization of a solution of bromine in CCl 4, is a useful visual test for unsaturation (double or triple bonds). Since both 1-hexene and 1-hexyne decolorize bromine, a second visual test is necessary to distinguish them from one another. webHomem-Formiga e a Vespa: Quantumania - Apple TV (PT) Disponível em iTunes, Disney+. Scott Lang (Paul Rudd) e Hope Van Dyne (Evangeline Lilly) regressam para .
{plog:ftitle_list}
webTá na Mídia - seu portal dos famosos. Erro: ID inválido fornecido na URL.
In any event, the decolorization of a solution of bromine in CCl 4, is a useful visual test for unsaturation (double or triple bonds). Since both 1-hexene and 1-hexyne decolorize bromine, a second visual test is necessary to distinguish them from one another.Reaction of bromine with alkenes or alkynes occurs through an electrophilic addition mechanism. The brown colored bromine reagent becomes incorporated into the organic .
Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The double bond breaks, and a bromine atom . • In the test tube, dissolve a few drops or a small amount of the compound in 2 mL of organic solvent. • Add the bromine reagent to the solution of the compound drop by drop .Bromine Test. A solution of bromine in \(\ce{CH_2Cl_2}\) is a test for unsaturation (alkenes and alkynes) and in some cases the ability to be oxidized (aldehydes). The bromine solution is orange and upon reaction the solution .
Br 2 (aq) is an orange or yellow solution, called bromine water and this is the halogen most commonly used. The unknown compound is shaken with the bromine water. If the compound is unsaturated, an addition reaction will take .Bromine water testing is an essential method for determining the presence of any alkene/alkyne functional groups in a chemical. Enols, anilines, alkenes, phenols, acetyl groups, and glucose .
Here are some common tests for each: ### Test for Alkenes: 1. **Bromine Water Test (Bromination Test):** - **Procedure:** Add bromine water (\ ( \text {Br}_2 \)) dropwise to .
When dissolved in a solvent, it forms an orange-brown solution. We can use bromine water to test for alkenes. When we add bromine water to a compound containing a double bond, an addition reaction takes place.This allows us to distinguish alkenes from alkanes using a simple chemical test. Bromine water is an orange solution of bromine. It becomes colourless when it is shaken with an alkene.
electrophilic alkenes with bromine
conclusion of hardness test
Bromine is used to test the presence of a double bond (alkene) in a molecule, reacting with the double bond in an addition reaction known as halogenation. A color change from yellow to colorless indicates the presence of a double bond. Explanation: Bromine (Br2) is utilized for testing the presence of a double bond, or alkene, in a molecule. An .The second test that was conducted to differentiate and classify our potential alkenes was the bromine test with the Br2/CH2Cl2 solution. Solutions with Br2 have an intense, deep red-orange color; however in the presence of alkenes, .
Click here 👆 to get an answer to your question ️ Bromine test may be used to detect the presence of unsaturated compounds of alkene and alkyne. . Bromine test may be used to detect the presence of unsaturated compounds of alkene and alkyne. Balance this reaction for bromine test below: Select one: A B. None of the above C D. Gauth AI .If bromine is spilled on the skin, flood the area with water for 10 minutes. You will be testing 4 different liquids: hexane, cyclohexene, toluene, and your unknown. In the hood, place 5 drops of each liquid to be tested in separate clean, dry test tubes. Label the tubes. Carefully add 3 drops of the bromine solution to each tube.2. Add a few drops of bromine water to the sample. 3. Observe the color change. If the solution turns colorless or light yellow, it indicates the presence of an alkene. The bromine test is a qualitative test for the presence of an alkene in an organic molecule. It involves adding bromine water to the sample and observing the decolorization of .
The bromine test is a chemical assay used to detect the presence of alkenes in a substance. In this test, the addition of bromine to an unknown compound causes a reaction that is indicative of the compound's saturation. If the compound contains alkenes, an addition reaction occurs and the brownish-orange color of the bromine solution will .Josie Beck 6/28/ Week 13: addition reactions of alkenes and alkynes Task: perform reflux on a fumaric acid + bromine solutionand color test an alkene, alkane, and alkyne Reaction: Name Structure Molecular weight (u) Density (g/mL) Mp / Bp (°C) Safety. Fumaric acid 116 1 298 / 355 Irritant. Bromine 159 3 -7 / 58 Corrosive, acute toxic, environmental hazard
conclusion of rockwell hardness test
Quiz yourself with questions and answers for Using bromine to test for alkenes, so you can be ready for test day. Explore quizzes and practice tests created by teachers and students or create one from your course material. . Test - Add bromine water Result - Orange solution turns to a colourless solution. 1 of 5. Term. what is the result if . Click here 👆 to get an answer to your question ️ 3) describe how would you experimentally test the presence of an alkene functional group. explain your answe. When bromine is added to a compound containing an alkene, the red color of bromine disappears. This color change indicates the presence of an alkene. Explanation: The bromine test is used to determine the presence of alkenes in a sample. When bromine is added to a compound containing an alkene, the reddish color of bromine disappears. The presence of an alkene can be determined using the 1) bromine water test, where the disappearance of the reddish-brown bromine solution color indicates a positive result for an alkene due to the addition reaction that forms a colorless dibromide. Explanation: To test for the presence of an alkene, the bromine water test can be employed.
To differentiate between an alkane and an alkene, you can use a simple chemical test called the bromine test.. Here's how it works: Obtain a small amount of the compound you want to test. Make sure you handle it safely in a well-ventilated area. Add a few drops of a bromine solution (usually a solution of bromine in an organic solvent like .PBP liberated bromine in the presence of alkenes/alkynes. t. PBP color is. orange brown, solution will then become colorless with alkene or alkyne present, which indicates a positive test addition product . an addition, positive alkene bromine solution test. KMON4 (aq) positive test indicates. color change from purple soluition to brown .
Yes, adding a small amount of bromine (Br2) solution can provide a test for the presence of an alkene. How does the bromine test indicate the presence of an alkene? A simple test with bromine water can be used to tell the difference between an alkane and an alkene.An alkene will turn brown bromine water colorless as the bromine reacts with the . The bromine solution, typically prepared in dichloromethane (CH2Cl2), is initially orange or reddish-brown due to the presence of bromine. However, when this solution is added to an alkene, the bromine reacts with the double bond, and the solution turns colorless, indicating a positive result for the test.VIDEO ANSWER: My opponent has 60,933,500 votes. I want to know how much more I won by rounding in the election. Let's round these two numbers to the nearest million. If I'm going to round to the nearest million, I need to know where the millions are
The bromine test is used to identify the presence of alkynes and alkenes by observing the decolorization of bromine's brownish-orange color, indicating an unsaturated hydrocarbon is present due to a halogenation reaction. Explanation: The bromine test is a qualitative analysis for the presence of alkenes and alkynes in an unknown organic compound.Ozonolysis is the cleavage of an alkene or alkyne with ozone to form organic compounds in which the multiple carbon-carbon bonds have been replaced by a double bond to oxygen. The outcome of the reaction depends on the type of multiple bonds being oxidized.In the bromine water test, a few drops of bromine water are added to the alkene. Bromine water is a solution of bromine in water, which has a characteristic orange-brown colour. If the alkene is unsaturated, it will react with the bromine water in a process called addition reaction. The double bond of the alkene breaks, and the bromine atoms . To test for the presence of an alkene, bromine water is used, which reacts with the alkene to decolorize the solution, indicating the presence of an unsaturated hydrocarbon. Explanation: The test that can be done for the presence of an alkene is the addition of bromine water, which is a brownish-orange solution of bromine.
Add 20 drops of water to a test tube labeled KMnO4 solution and add 1 drop of potassium permanganate. Set it aside for comparison with the test solutions. 2. Place 20 drops of each listed hydrocarbon in separate clean small labeled test tubes: alkane, alkene, aromatic, and unknown 3. Add 1 drop of KMnO4 to each test tube and shake well.
A positive bromine test appears as the disappearance of the orange color of the bromine solution, indicating that the bromine has reacted with an unsaturated compound like an alkene or alkyne. 3. A negative bromine test appears as the retention of the orange color, suggesting that the compound is saturated and that no reaction has taken place .
Add 2-3 drops of dried product to additional test tubes 3. add bromine solution to both test tubes. If alkene is present, the red color of the bromine solution will become discharged upon addition 4. add potassium permanganate to both test tubes. if alkene is present a brown solid will form upon addition
Study with Quizlet and memorize flashcards containing terms like The bromine test shows the presence of _____ A positive bromine test appears as_____ A negative bromine test appears as_____, Determine whether each description applies to saturated or unsaturated fatty acids. Contain carbon-carbon double bonds_____ Contain no carbon-carbon double bonds_____ . 2. reaction of chlorine or bromine in aqueous solution such as bromine water alkanes react in the presence of uv light so to cut out the uv light you can wrap your test tube in foil, like we did in our prac, or put it somewhere dark. i think you do have to shake them, but not shaking them doesn't mean they won't react.
Alkenes are unsaturated hydrocarbons. . The bromine test shows the presence of. alkenes. A positive bromine test appears as. a colorless solution. A negative bromine test appears as. an orange solution. Which structure shows the correct bonding for the product? Baeyer's test, also known as the permanganate test, shows the presence of . Prepare two test tubes. Add a few drops of bromine water to both of the tubes containing pentane and pentene, respectively. Observe! That solution that decolourizes is PENTENE. Alkene undergoes halogenation with bromine to produce dibromopentane. No reaction between pentane and bromine water, reddish-brown colour remains.
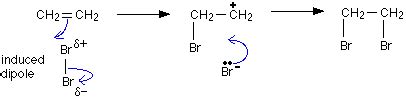
aqueous solution of bromine
concrete hardness scratch test
10 de jan. de 2023 · Asking questions in Pyhton entails asking a question and accepting an answer from the user. This is achieved using the input () function in Python 3 or raw_input () in Python 2. The question is passed to the function as , and the user’s input is accepted. Once the answer has been typed, the user can exit the .
drops of bromine solution to test presence of alkene bones|alkyline bromination test